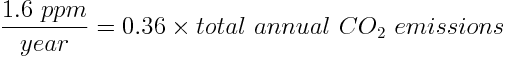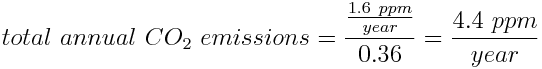Now we can calculate the increase in atmospheric carbon dioxide concentrations due to human activity. It is estimated that combustion of oil accounts for 36% of the total anthropogenic carbon dioxide emissions. Coal (40%), natural gas (20%) and other sources (4%) account for the rest of human carbon dioxide emissions.
Worked Example

What is the increase in atmospheric carbon dioxide concentration as a result of human activity over a one year period? Express the answer in ppm of CO2. Assume that the mass of carbon released from combustion of oil is approximately 3.4 gigatonnes of carbon per year, that combustion of oil accounts for 36% of human emissions of carbon dioxide and that the atmosphere contains about 1.8x1020 moles of gases.
First, calculate the amount (in moles) of carbon dioxide released into the atmosphere each year due to the combustion of oil.
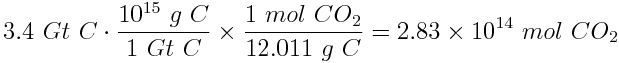
We can now calculate the annual fraction of CO2 contributed from oil combustion relative to the entire atmosphere, assuming the atmosphere contains about 1.8x1020 moles of gases:
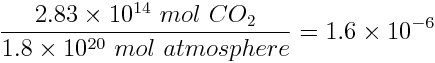
To express this ratio of CO2 to the whole atmosphere in ppm, the ratio must be multiplied by 1 million. We find that the concentration of CO2 released into the atmosphere from the combustion of oil is 1.6 ppm/year.
However, we need to take into consideration the other sources of anthropogenic CO2 in order to calculate humanity's total yearly carbon footprint. We know that the 1.6 ppm of CO2 released from combustion of oil makes up around 36% of total yearly CO2 emissions. This can be used to calculate the total yearly anthropogenic CO2 emissions: