Isotopes are used to determine historical temperatures. Oxygen is an element that is commonly used in isotopic studies and oxygen atoms are found in nature as one of three stable isotopes: oxygen-16, oxygen-17 and oxygen-18. Depict each of these isotopes in symbolic form.
Remember, in symbolic form, the atomic number (Z) is subscripted and the mass number (A) is superscripted, followed by the symbol of the element. In symbolic form, oxygen-16 is , oxygen-17 is
and oxygen-18 is
.
Your Turn
An understanding of isotopes has allowed scientists to obtain important data in studies of climate as well as other scientific disciplines. Give the names and symbols of the following nuclei. Which of these nuclei depict isotopes of the same element?
 Proton
Proton
 Neutron
Neutron

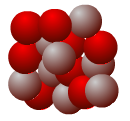

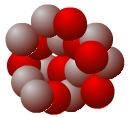
Nuclei B and D are isotopes of the same element because they have the same number of protons but different numbers of neutrons. Nucleus B is oxygen-16 (16O), while nucleus D is oxygen-18 (18O). Nucleus A only has 7 protons, and is nitrogen-14 (14N). Nucleus C has 9 protons and is fluorine-19 (19F).