Evidently, the high specific heat capacity of water can have drastically different effects on climate: dependant on geographic and seasonal conditions the oceans can either moderate temperatures, or increase the intensity of violent storms. However, in addition to these climate impacts, water’s high specific heat capacity has important implications when considering the consequences of climate change.
By emitting greenhouse gases, humans are increasing the temperature of the atmosphere. As the temperature of the atmosphere increases, the atmosphere can then release more energy as heat to the oceans. Because the oceans are so large and because water has a high specific heat capacity, the ocean is capable of absorbing a huge amount of energy without undergoing an increase in temperature. In fact, the ocean continues to absorb more than 90% of global warming.
Worked Example
As the temperature of the atmosphere warms due to climate change, the oceans can absorb some of this energy, so that the atmosphere’s temperature does not increase as much as it would otherwise.
The volume of the world’s oceans is approximately 1.3×1021 L. Assuming that the specific heat capacity of seawater is 4.18 J K-1 g-1, how much heat can the world’s oceans absorb before increasing by 1 K?
One liter of pure water has a mass of 1000 g. Assuming that the density of seawater is close to that of pure water, the mass of the world’s oceans is:
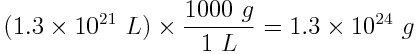
Now, calculate the absorption of heat that accompanies a temperature change of 1 K for 1.3×1024 g of water.
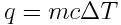
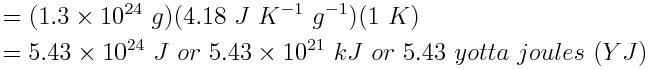
Note that this is only a rough approximation of how the ocean absorbs energy. In reality, the top portions of the ocean absorb most of the energy, and very little energy reaches ocean depths over short time frames.