Scientists use the specific heat capacities of different substances to calculate the amount of heat associated with a given temperature change, using the following equation:
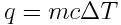
In the equation, q is the amount of energy transferred (heat) to cause the observed temperature change. The units of q are joules, J, or kilojoules, kJ, which are commonly used units for energy measurements. m is the mass of the substance (in grams), c is the specific heat capacity of the substance (in J K-1 g-1) and ΔT is the change in temperature experienced by the substance (in Kelvin, K). If the temperature of a substance increases, T > 0, energy must have been transferred to the substance and the heat, q, is positive. However, if the temperature of a substance decreases, T < 0, energy must have been transferred away to another object and the heat, q, is negative.
We can use this equation to estimate how much heat is transferred to a certain amount of water when submerging a hot rubber stopper.
Worked Example
A hot rubber stopper is submerged in 500 mL of water and the temperature of the water increases from 20 °C to 24 °C. The specific heat capacity of water is 4.18 J K-1 g-1.
- Calculate the amount of heat transferred to the water.
- How much heat was released by the rubber stopper?
-
The mass of 500 mL of water is 500 g, because the density of water is 1 g mL-1. Use the equation for calculating the heat flow associated with a given temperature change. The change in temperature must be expressed in Kelvin, but a change of 1 °C is equal to a change of 1 K, so the temperature change of +4 °C is simply +4 K.
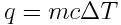
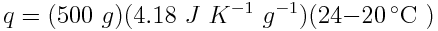
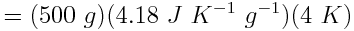
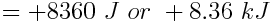
The water absorbed 8.36 kJ of heat. Because this value is positive, we know that the water absorbed, rather than released, the heat.
- The heat absorbed by the water was released by the rubber stopper. Therefore, the amount of heat released by the stopper is equal in amount to the heat absorbed by the water, but opposite in sign. The stopper released -8.36 kJ of energy as heat.