The ocean is one of the most important carbon sinks. More than 2 Gt of anthropogenic carbon dioxide in the atmosphere is taken up by the ocean every year. What happens to this carbon dioxide that enters the ocean? How can chemistry contribute to our understanding of the environmental cost of this uptake?
What Do We Know?
When carbon dioxide enters the ocean, it takes part in various chemical reactions. One important series of reactions involving carbon dioxide in the oceans is shown below:
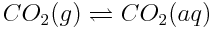

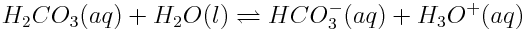
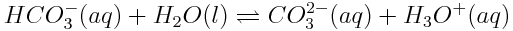

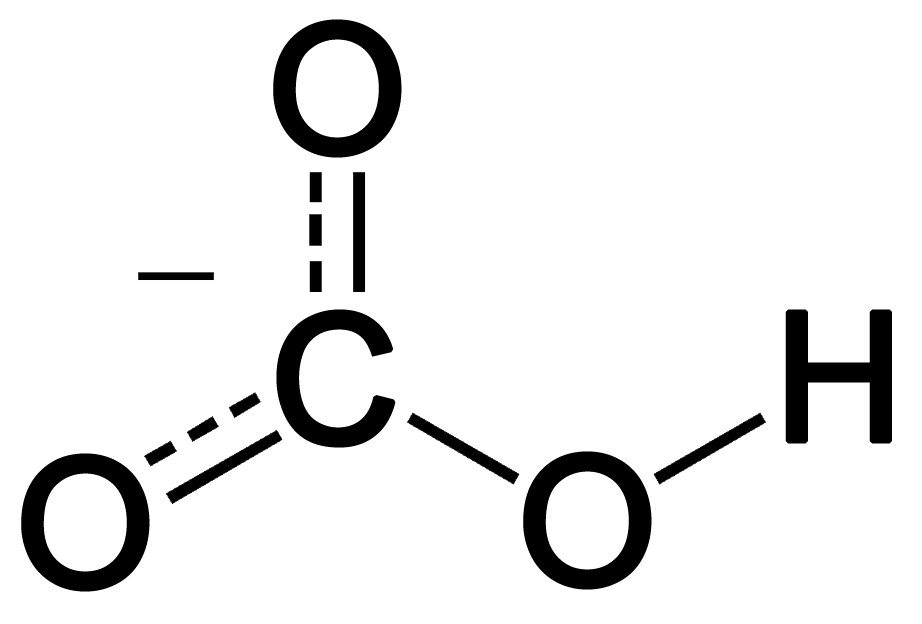
The net effect of these reactions serves to lower the ocean’s pH, making it slightly more acidic (more correctly, less basic) than it has been. Although other substances in the ocean, specifically aquated borate ions, B(OH)4-(aq), play a very significant role in determining ocean pH, the substances shown above are causing the overall pH balance of the oceans to shift.
Open the Carbon Dioxide and Ocean pH Learning Tool to learn how the pH of the ocean responds to changes in the concentration of carbon dioxide in the atmosphere. Adjust the slider bar controlling the concentration of atmospheric carbon dioxide and observe how the pH of the ocean changes.
Worked Example
The current concentration of atmospheric carbon dioxide is about 400 ppm. Using the Carbon Dioxide and Ocean pH Learning Tool, what is the pH of surface ocean water at this carbon dioxide concentration?
Adjust the Atmospheric CO2 slider bar to 400 ppm.
At the current concentrations of carbon dioxide in the atmosphere, the pH of the ocean is 8.05.
Your Turn
Human activity is increasing the concentration of carbon dioxide in the atmosphere. If the concentration of atmospheric CO2 increases to 600 ppm, what will the pH of the surface ocean be? Use the Carbon Dioxide and Ocean pH Learning Tool.
At 600ppm on the Atmospheric CO2 slider bar, the pH of the ocean is calculated to be 7.89.
What is the significance of a drop in pH of 0.16 pH units? We will address this later in the lesson.