The equilibrium reactions involving atmospheric carbon dioxide also affect the speciation of carbon in the ocean. This simply refers to the specific chemical form in which carbon is found. In the ocean, carbon atoms may be found in a variety of forms, including dissolved carbon dioxide (CO2), carbonic acid (H2CO3), hydrogen carbonate (or bicarbonate) ions (HCO3-), carbonate ions (CO32-) or calcium carbonate (CaCO3). Note, however, that carbonic acid is an unstable molecule, and exists in essentially negligible quantities in the ocean. Most of the carbonic acid produced reverts back to dissolved carbon dioxide. However, since the equilibria of CO2(aq) and H2CO3(aq) are so interdependent,"carbonic acid" will be used to describe both H2CO3(aq) and CO2(aq). Reactions in the ocean change the concentrations of these various carbon species. These changes in concentration can have important effects on marine life in the ocean because marine organisms use carbonate ions to build their shells.
Return to the Carbon Dioxide and Ocean pH Learning Tool and click the 'Show Graph' button to see how the concentrations of carbonic acid, hydrogen carbonate (bicarbonate) and carbonate change as the concentration of atmospheric carbon dioxide is changed.
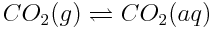

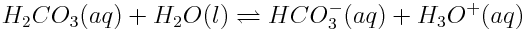
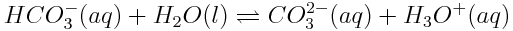
In the graph in the Carbon Dioxide and Ocean pH Learning Tool, the blue line shows the concentration of carbonate ions. If the concentration of atmospheric carbon dioxide increases, how does the concentration of carbonate ions in the ocean change? Why do you think this change occurs? How will a change in ocean carbonate ion concentration affect marine life?