As you can tell from the previous worked example, the variation in isotopic abundances across the Earth is very small; the percent abundance of chlorine-35 in nature typically only varies from approximately 75.555% to 76.085%. However, this variation is significant and can be measured using sophisticated high-resolution mass spectrometers. For instance, two samples containing chlorine atoms from distinct locations will have different chlorine-35 and chlorine-37 abundances, producing different peak intensities at 37 m/z and 35 m/z in a sensitive mass spectrum. Such differences in isotopic abundances result in the variations in atomic weights.
Your Turn
Challenge Question: Nitrogen, which plays an important role in the chemistry of the atmosphere, is given an atomic weight interval from 14.00643 to 14.00728 by the IUPAC Periodic Table of the Isotopes. Calculate the percent abundances of the nitrogen isotopes, which produce this interval of atomic weights. The relative atomic mass of nitrogen-14 is 14.003074 and relative atomic mass of nitrogen-15 is 15.000108. Use the Atomic Weight Calculator to check that the abundances which you calculate result in the interval of atomic weights given by the Periodic Table of the Isotopes.
The percent abundance of nitrogen-14 given by an atomic weight of 14.00643 is:
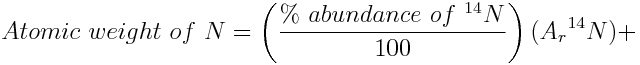
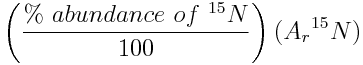
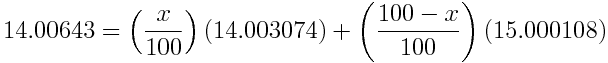
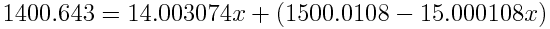
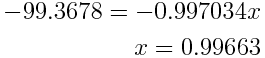
If the atomic weight is 14.00643, the percent abundance of nitrogen-14 is 99.6634% and the percent abundance of nitrogen-15 is 100%-99.6634%=0.3366%.
Therefore, the atomic weight interval given by the IUPAC Periodic Table of the Isotopes is produced by a range of percent abundances from 99.5781% to 99.6634% for nitrogen-14 and from 0.3366% to 0.4219% for nitrogen-15.
In the Atomic Weight Calculator , enter the percent abundance values for nitrogen-14 and nitrogen-15 which you calculated. Do these values result in the atomic weight interval for nitrogen that is given in the IUPAC Periodic Table of the Isotopes?